Cross-Topic Integration (15 of 26)
The oxidation-reduction system, which looks at a chemical reaction as a narrative of electron control, is basically an accounting system to keep track of the tendency for bonds with very electronegative atoms to be stronger bonds. An electron in such a bond has fallen into a deep potential energy well.
In the context of covalent bonding, the reduction potential of an element depends primarily on its electronegativity. The more electronegative an atom, the greater the internal energy decrease involved in bond formation as it pulls electrons towards itself. Electronegative elements love to gain electron control and form strong bonds.
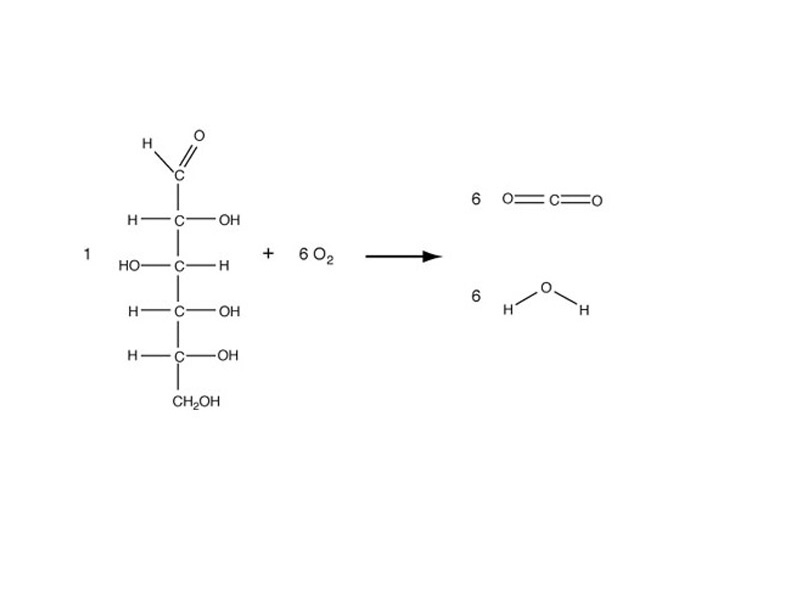
Looking at a oxidation-reduction reaction, such as combustion of glucose, in terms of bond dissociation energies to build our thermodynamic perspective is actually more conceptually transparent than the redox system, but the redox system is more convenient. It uses its accounting perspective to give you the same answer.
Somewhere along the line in the history of science education, though, these two systems of meaning seem to have detached from each other in the basic teaching, and not too many students learn the underlying rationale for the oxidation-reduction system. In biology, for example, there is constant reference to 'high energy electrons' when the understanding would greatly benefit from not only an oxidation-reduction approach but also Hess' Law.