Cross-Topic Integration (16 of 26)
It is proper under Hess' Law of Heat Summation, in chemical thermodynamics, to imagine a simplified pathway for a chemical process to understand the changes in thermodynamic state functions. Let us reprise our imaginary thermochemical path for the solution process.
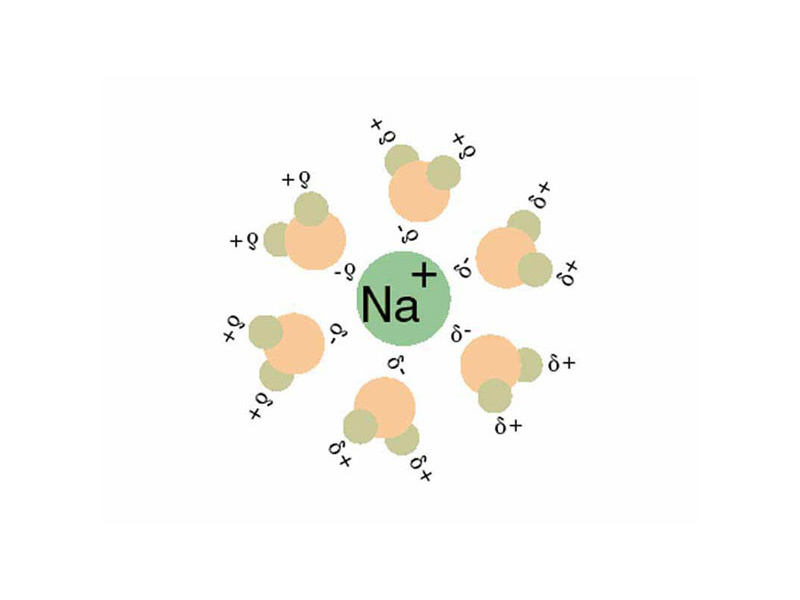
An imaginary path from pure solute and solvent to solution can be conceptualized by increasing electric potential energy along lines of intermolecular force as the molecules of solvent and solute are separated. This is followed by decreasing potential energy as the molecules come together to form the solution.
In addition to net internal energy change (which equals the enthalpy change in transformations where the total volume changes little or not at all, as in solution processes generally) the equilibrium will also be determined by the effects of changes in the state of disorder in the system, or entropy. For nonpolar solvents dissolving nonpolar solutes, entropy generally increases, while for electrolytes dissociating in water, entropy usually decreases. For salts dissolving in water, entropy usually decreases. This is because of the order imposed on the water through the formation of hydration shells.